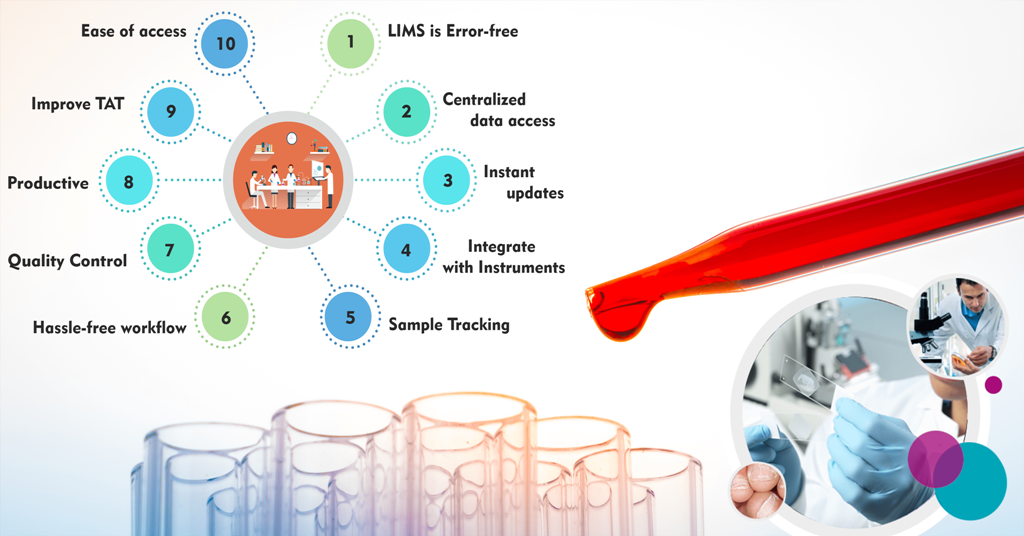
MocDoc's Offerings
What is laboratory information management system?
Published By
Sanjana
2018080807:57:04
Category LIMS

An Introduction to LIMS Software:
Laboratory Information Management System (LIMS) is software that is used in labs for data management and to process a large number of lab samples to manage laboratory workflow. With LIMS, the lab can automate workflows, integrate instruments, and manage samples and associated information. Additionally, labs can produce valid results quickly and can track data across experiments to improve efficiency.
LIMS that are commonly used in clinical labs fall under the following categories:
- Clinical Chemistry Analyzers
- Diagnostic Testing
- Immunoassay Systems etc.,
The benefits of using LIMS Software in your lab are:
- Workflow automation
- Integrate instruments or other in-lab systems
- Centralize access and storage of data
- Track data from sequencing runs
- Initiate downstream data analysis
The Efficiency of LIMS System:
The main purpose of using the LIMS System in a lab is to improve the efficiency of sample processing and management. Using LIMS would cut down on the need for manual tasks thereby increasing the accuracy of sample analysis. LIMS captures data automatically, gets processed, and is stored for future reference. The LIMs vendor you choose will define the proceeding process of the software.
Generally, the process of a LIMS is divided into five stages:
- The sample is logged in after reception.
- Assignment, scheduling, and tracking of the sample and the analytical workload.
- Processing and quality control associated with the sample.
- Storage of data associated with the sample analysis.
- Inspection, approval, and compilation of the sample data for report generation and further analysis.
Types of LIS Modules Used In Clinical Laboratory:
The features of Laboratory Information System (LIS) have seen evolutionary growth over the years from sample tracking to enterprise resource planning for clinical and specialty laboratories.
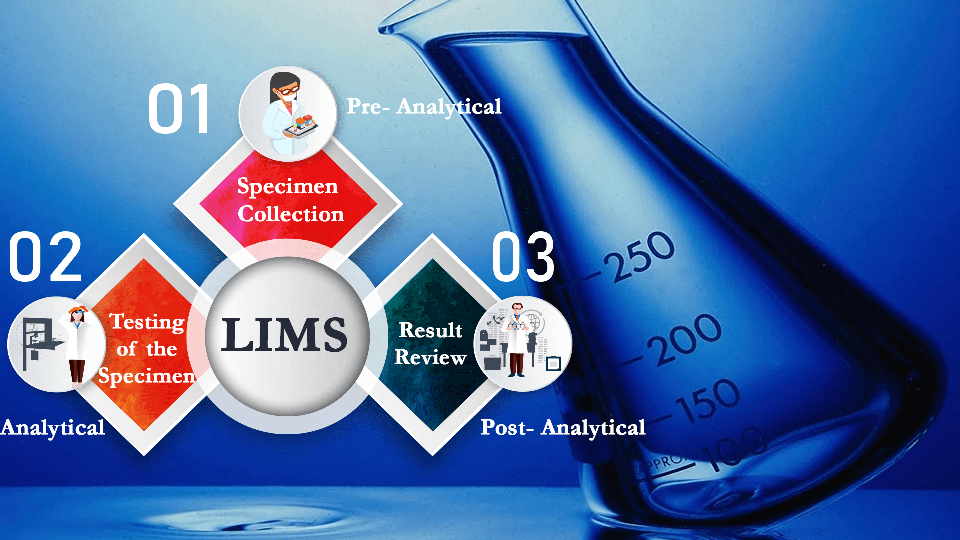
A typical workflow pattern of a LIS in a clinical lab flows through three phases:
- Pre-analytical: Specimen collection.
- Analytical: testing of the specimens.
- Post-analytical: Result review.
The LIS modules are used by various laboratory departments like:
- Blood Bank
- Immunology
- Hematology
- Microbiology
- Clinical chemistry
- Histopathology
Various Common Functionalities Used In LIMS:
With the progress in technology, the functions of a LIMS progress too. LIMS software is built in a way to support any upcoming progress while effectively working on a basic set of definite functionalities like the collection of data, tracking of samples, processing, data storage, and report generation.
Some of the core functionalities of LIMS are explained below:
Sample Management:
LIMS was created to help in the process of sample management systems occurring in laboratories. This system gets initiated when a sample is received in a laboratory and gets registered in a LIMS. Since a large number of samples are collected and analyzed in a day, it would be difficult to record the samples without the chance of being confused with other analyses. With LIMS, It would be easier to track the sample if it is labeled and assigned correctly by using RFID on the samples or barcodes.
The information collected and stored via a LIMS would fall under the following criteria:
- Details about the person whose sample is taken.
- Details of the doctor/clinic who recommended an analysis.
- The tests/analysis needed to be taken on the sample.
- Stages of the sample and destination.
- Storing procedures.
- Expected report generation date.
- Record creation for future purposes.
Workflow Management:
The need for workflow management using a LIMS is as important as Sample management and record-holding. The accuracy of a working system does not just depend on the data; it needs an on-point process assessment where a LIMS system can be used. As the name indicates, workflow management helps automate workflows and assign work if delegated precisely in the software.
All you would have to do for better workflow management using LIMS is:
- Enter proper codes to direct the LIMS system for a work assignment.
- Configure the process flow of the work for sample analysis.
- Assign exact instruments for the particular work.
Workflow management using a LIMS saves time and conducts the job with precision.
EMR/EHR Software:
Electronic Medical Record (EMR) / Electronic Health Record (EHR) is software that collects and stores information on the patient’s medical history in the form of records that can be shared via different healthcare mediums. Many LIMS have this EMR/EHR Software functionality built-in in their system. Using a LIMS with an EHR function will be a huge asset to your lab as the real-time data exchange can be made possible easily.
Related Articles
Is Cloud-Based SaaS Laboratory...
In every industry, modern laboratories are under p..... Read more
Streamlining Sample Tracking w...
Laboratory Information Management System is ..... Read more
Why should you invest in a Lab...
A Laboratory Information Management System (LIM..... Read more
Why Many laboratories Fail And...
Going wrong in experiments taking place in laborat..... Read more