MocDoc's Offerings
Streamlining Sample Tracking with MocDoc LIMS
Published By
Preethi
2023022316:26:27
Category LIMS
Laboratory Information Management System is an extensive solution, designed to cater for the needs of the medical testing labs, and facilitates seamless monitoring of the lab’s operations by streamlining the processes.
The demand for medical lab services is high and keeps increasing with time, which makes it critical to ensure efficient and high-quality customer service. A sample management system is required to handle this necessity. An appropriate Sample Tracking system guides a medical examiner in streamlining the sample lifecycle, monitoring and operating the laboratory processes, with its comprehensive overview of the test sample. This ensures that nothing is overlooked, from sample collection to movement, to arriving at the test results, by tracking the entire workflow and recording the status.
MocDoc’s reliable laboratory management software performs the sample tracking task, maintaining quality standards and also helping to improve operational efficiency.
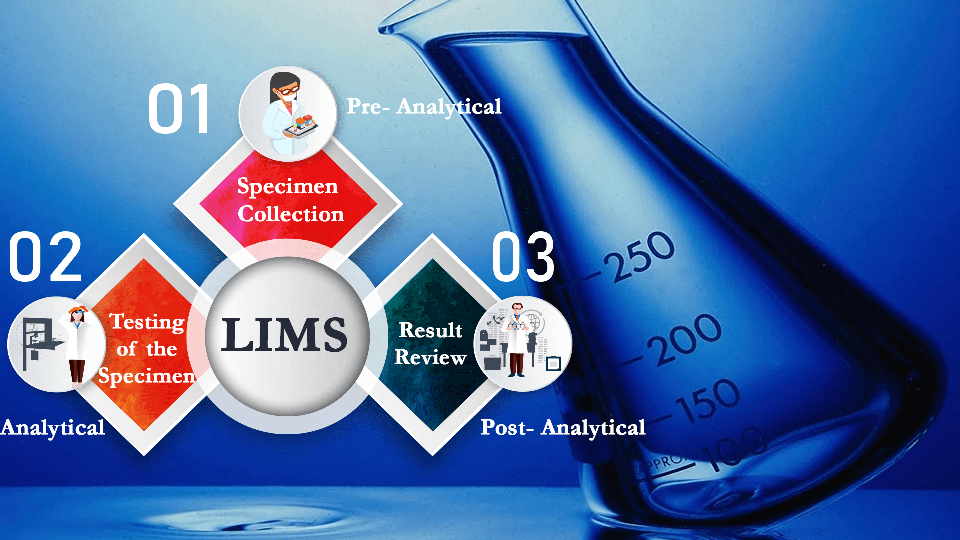
The Sample Lifecycle:
The Sample Lifecycle is an integral part of laboratory operations and encompasses three distinct phases: Pre-Analytical, Analytical and Post-Analytical. Managing the sample lifecycle effectively is essential to ensure accurate results and avoid any leaks or errors due to poor sample management.
● Pre-Analytical phase: The Pre-Analytical phase begins when a test order is placed and the sample is collected. The sample goes through the process of accession, where it is acknowledged or rejected for testing. If the sample is accepted, it is then segregated based on the type of sample and the department
● Analytical phase: The Analytical phase is a step of utmost importance, in the sample lifecycle, where the collected and segregated sample undergoes the ordered test and results are generated. In this phase, the laboratory staff will be responsible for selecting the appropriate test methods, preparing the samples, running the tests and ensuring that the recorded results are accurate, precise and consistent.
● Post-Analytical phase: The Post-Analytical phase is the final and crucial step in the sample lifecycle, where the test results are reviewed and verified for accuracy and completeness by a professional, such as a clinician or a doctor, and any necessary corrections or amendments are made. The final report is then generated and delivered to the patient.
The special features of the MocDoc LIMS Sample Tracking system:
Monitoring a sample’s progress throughout its lifecycle helps the professionals in managing the laboratory processes effectively, by analyzing the duration it spends in each department. This enables labs to maintain a satisfactory TAT consistently and also facilitates identifying the cause of any testing delay.
Sample Recieve Workflow:
The Sample Receive Workflow feature in MocDoc LIMS streamlines the process of receiving samples in a laboratory. Sometimes, the samples collected from the patient in the accession department may not reach the processing unit on time, hence it is important to track the sample's arrival time for maintaining allocation turnaround time. With a simple barcode scan, technicians can efficiently receive and track the sample's arrival time, ensuring that the testing process remains within the allocated turnaround time (TAT). This feature is essential for accurately monitoring the progress of samples and ensuring that the results are produced on time.
Staged Process Workflow:
The testing process carried out for a few samples may involve various stages such as cassette preparation, and specimen staining, before the final test results are obtained. These processes can vary depending on the type of test being conducted. It is crucial to monitor these processes to determine the current status of the sample and the time taken for each stage of processing.
This is accomplished with the "Staged Process Workflow" in MocDoc, which tracks the progress of each test at every process, keeping a record of the sample's journey from preparation to completion. The workflow also provides insights into the time taken for each intermediate stage. Customized workflows can be created for each test, based on its specific requirements.
With the advent of technology, the MocDoc LIMS System has made the process of tracking samples simpler. MocDoc's Sample Tracking module allows you to track the status of the samples throughout their lifecycle, from the time of collection to the time of archival, which in turn helps the labs maintain their brand reputation and service standards
Related Articles
Why Machine Interfacing in LIM...
As labs are updated and become more modern, better..... Read more
Using LIMS for Quality Control...
Keeping up with the fast-evolving world of trends ..... Read more
What is laboratory information...
..... Read more
Complex machine integration wi...
Save time on complex machines at your lab with Moc..... Read more