MocDoc's Offerings
Why Machine Interfacing in LIMS is important
Published By
Sanjana
2018092708:52:44
Category LIMS
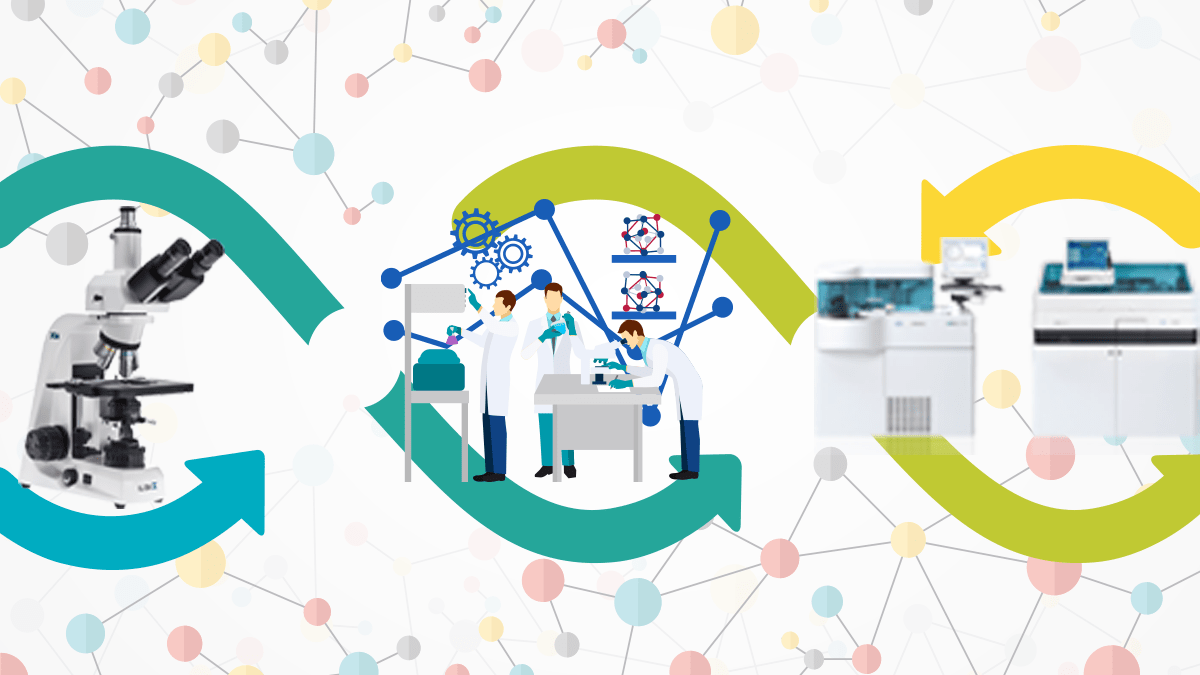
As labs are updated and become more modern, better methods of data management and record-keeping may be necessary to maintain or improve efficiency. Luckily, there is a means to achieve this: Laboratory Information Management Systems, or LIMS.
With the increase in disease and the growth in the medical diagnosis sector, the number of samples collected is increasing day by day. With the help of Machine interfacing, labs can handle a large volume of samples easily, increasing the efficiency of sample processing and the quality of lab reports.
Types of Machine Interfacing in Laboratory Information Management System(LIMS):
Uni-Directional Instruments:
Overview: Uni-directional instruments operate on a one-way communication system, facilitating the seamless transfer of information from the samples to the Laboratory Information Management System (LIMS). In this setup, results are stored within the LIMS database upon the completion of the test process. Unlike bi-directional instruments, which support two-way communication, uni-directional instruments focus on unidimensional data flow.
Workflow Process: The workflow involves manual sample placement by the technician, who assumes the responsibility of strategically positioning samples for analysis. Additionally, the technician plays a crucial role in determining the specific type of sampling method to be employed. This hands-on approach ensures that the instrument receives accurate and relevant data for subsequent processing.
Result Storage: Upon completion of the test, the results are systematically stored in the LIMS database. This unidirectional flow of information simplifies data management, with the LIMS acting as a centralized repository for comprehensive result storage. This streamlined process contributes to the efficiency of data retrieval and analysis within the laboratory setting.
Technician Involvement: The technician's involvement is pivotal in the uni-directional instrument setup, as they guide the initial placement of samples and make informed decisions about the sampling technique. This level of manual intervention ensures precision and control over the testing process, aligning with the laboratory's specific requirements and protocols.
Uni-directional instruments, by emphasizing one-way communication and manual input from skilled technicians, contribute to a structured and controlled laboratory environment where data accuracy and integrity are paramount.
Bi-directional Instruments:
Advanced Functionality: Bi-directional instruments represent a higher level of sophistication and advancement in laboratory technology. These instruments possess the capability to not only generate output data reports and results but also feature an Application Programming Interface (API). This advanced functionality enables the seamless exchange of data, information, commands, functions, and worklists with the system.
Output Data Reports: One key feature of bi-directional instruments is their ability to generate comprehensive output data reports. These reports provide a detailed analysis of test results and relevant information, offering a comprehensive view of the findings. The generated reports serve as valuable documentation for further analysis and decision-making processes.
Results Generation: Bi-directional instruments excel at producing accurate and timely results. The dual communication capabilities allow for efficient data transfer, ensuring that results are not only generated promptly but also transmitted seamlessly to the designated system or database.
Application Programming Interface (API): A defining feature of bi-directional instruments is the inclusion of an Application Programming Interface (API). This interface facilitates the exchange of data, information, and commands between the instrument and the broader laboratory system. The API functionality enhances interoperability, enabling the instrument to import and export data with ease.
Enhanced Connectivity: Bi-directional instruments contribute to enhanced connectivity within the laboratory ecosystem. The ability to import data, information, commands, functions, and worklists through the API fosters a dynamic and integrated environment. This connectivity streamlines laboratory processes, promoting efficiency and accuracy in data handling.
Our LIMS System sends commands to the sampling unit on the type of sampling to be conducted with the given sample. After the testing is done by the LIMS command, the results are auto-fetched on the LIMS Server. The bi-directional instruments are interfaced with the LIMS system programmatically.
The Need to Interface Instruments with LIMS Software:
There are several reasons why it is beneficiary to interface lab instruments with LIMS Software. Let us look into a few reasons here:
Increase in Productivity by Effective Machine Utilization:
Interfacing your instruments with your LIMS Software is a one-time investment of cost and work with a repetitive increase in lab productivity and effectiveness. Interfacing LIMS Software with your lab instruments removes many complexities like manual entering of data every time or referring data from other instruments to the LIMS. This altogether saves a lot of time and effort when done by a LIMS rather than manually, also avoiding any human errors during data entry.
Utilizing machines effectively can increase productivity drastically. When the task is done manually, very few samples are completely analyzed in the given period, but with MI they can perform many sample processing and that will increase machine utilization and increase revenue.
Increase in Data Quality by Reducing Manual Errors:
The second best reason for interfacing your lab instruments with your LIMS Software is the increase in data quality and integrity. As a human, we are eligible to commit human errors that might not be over-influential over the system as a whole but might still be considered an error. By removing human interference, these errors are avoided thereby guaranteeing better data transfer in the system.
Similarly, transcribing data into the system by people can also lead to common errors affecting the quality of data. Data integrity is considered a very important issue in laboratories. By interfacing your instruments to your LIMS System, you will eliminate this issue.
Saves Time in output generation thereby satisfying the patients:
Machine Interfacing helps to analyze the given samples at a faster rate and generates quality results within a shorter duration of time. After the result processing is done, the report will be generated manually.
With the use of MI, ultimate patient satisfaction can be achieved by generating the results in a quick manner with good quality.
Machine interfacing in laboratories is more of a comfort rather than a negative factor. What might be a one-time investment in your labs can save a lot of time and work when interfaced properly with your systems.
Related Articles
Is Cloud-Based SaaS Laboratory...
In every industry, modern laboratories are under p..... Read more
Revolutionize B2B sample colle...
MocDoc’s Runnr mobile/web application is designe..... Read more
Why Many laboratories Fail And...
Going wrong in experiments taking place in laborat..... Read more
Why should you invest in a Lab...
A Laboratory Information Management System (LIM..... Read more