MocDoc's Offerings
Why should you invest in a Lab Information Management System?
Published By
Sowmya
2018053118:00:18
Category LIMS


A Laboratory Information Management System (LIMS): Why Do You Need One?
The adoption of Laboratory Information Management System (LIMS) has become increasingly crucial. This comprehensive guide delves deep into the reasons behind investing in LIMS, shedding light on the myriad benefits it offers to laboratory operations. As we explore the multifaceted facets of LIMS, you'll discover how this powerful tool revolutionizes lab management, enhancing precision, efficiency, and overall quality. Whether you're running a pathology lab, clinical research facility, or any laboratory, this article is your key to understanding why LIMS is a game-changer.
Chapter 1: LIMS Software - A Paradigm Shift in Lab Management
In this digital age, traditional manual processes in laboratories have become a bottleneck, prone to errors and inefficiencies. LIMS software, also known as a laboratory management system, emerges as a game-changer, transforming how laboratories operate. It encompasses a wide range of functionalities, including ERP tools, data analytics capabilities, and virtual software. Let's explore why LIMS is gaining ground in modern laboratories.
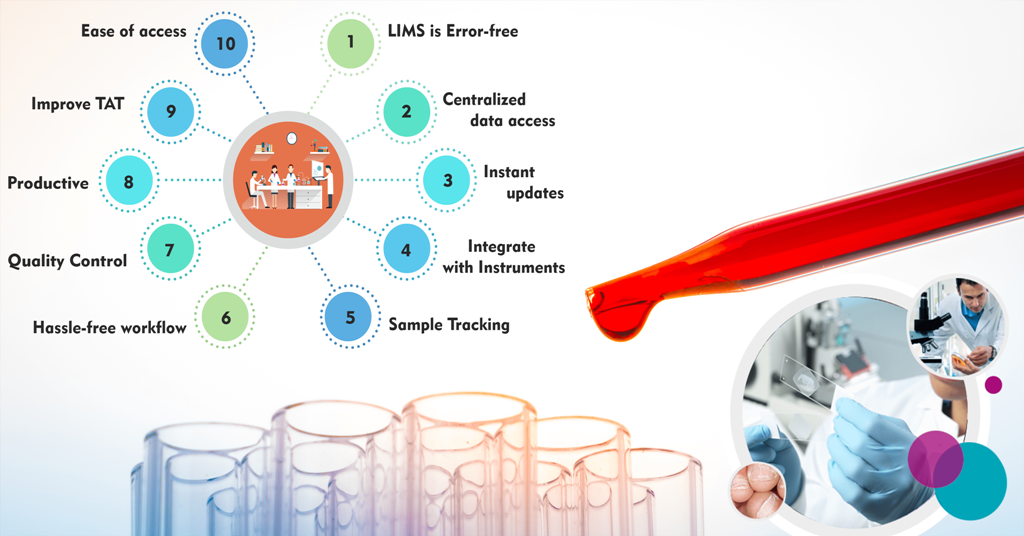
1.1. Error-Free Precision
LIMS software is designed to be virtually error-free, a crucial feature for laboratories dealing with sensitive data. Manual handling of laboratory tasks often leads to data mismatches, incorrect readings, and erroneous updates. LIMS ensures impeccable record precision, eliminating these sources of error.
1.2. Centralized Data Access
One of the standout features of LIMS is its ability to provide centralized data access. This means that patient records and reports are easily accessible to authorized personnel. Clinicians, technicians, and other stakeholders can quickly reference and verify patient details. Moreover, LIMS automatically updates critical values and responds to crises, ensuring immediate attention to critical cases.
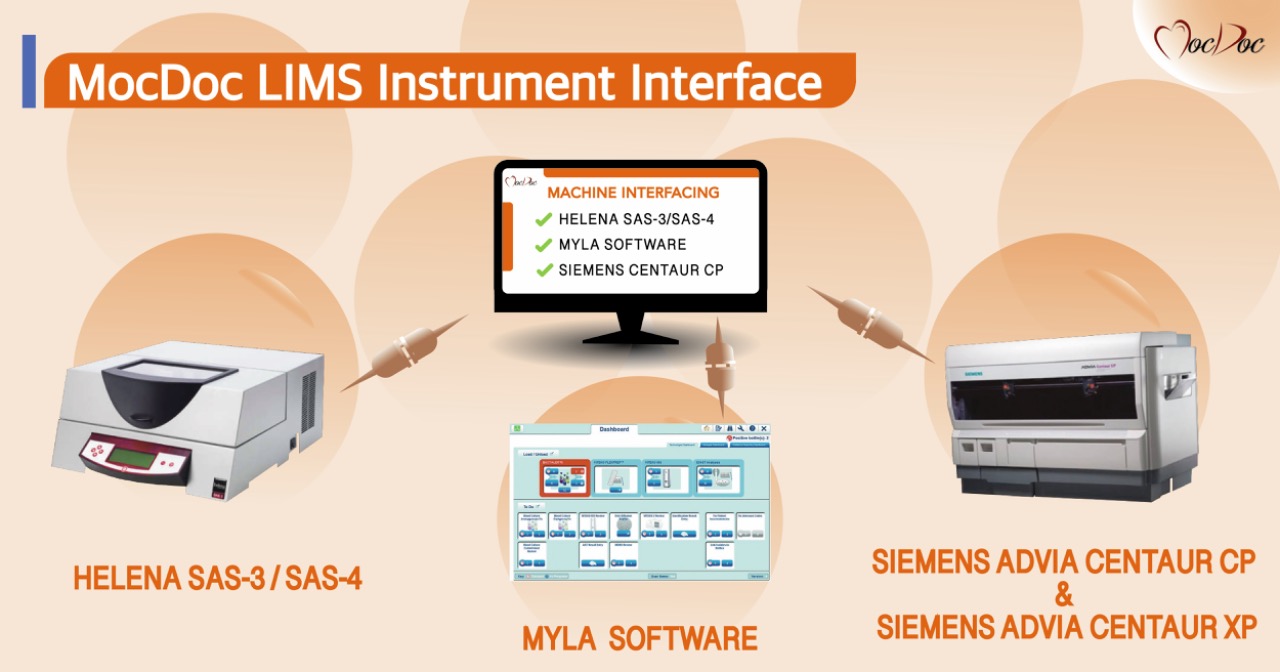
1.3. Seamless Integration with Instruments
LIMS seamlessly integrates with clinical instruments, streamlining the testing process. It automatically retrieves and stores test results in its primary database, eliminating the need for manual data entry. This not only reduces the risk of errors but also saves valuable time.
Chapter 2: Enhancing Patient Experience through LIMS
2.1. Patient-Focused Updates
LIMS introduces a new level of patient engagement by providing real-time updates. Patients no longer need to anxiously wait for their test results. Instead, they receive automatic updates via SMS or email, along with concise test result summaries. This not only enhances patient satisfaction but also ensures they are well-informed about the progress of their tests.
2.2. Sample Tracking for Quality Assurance
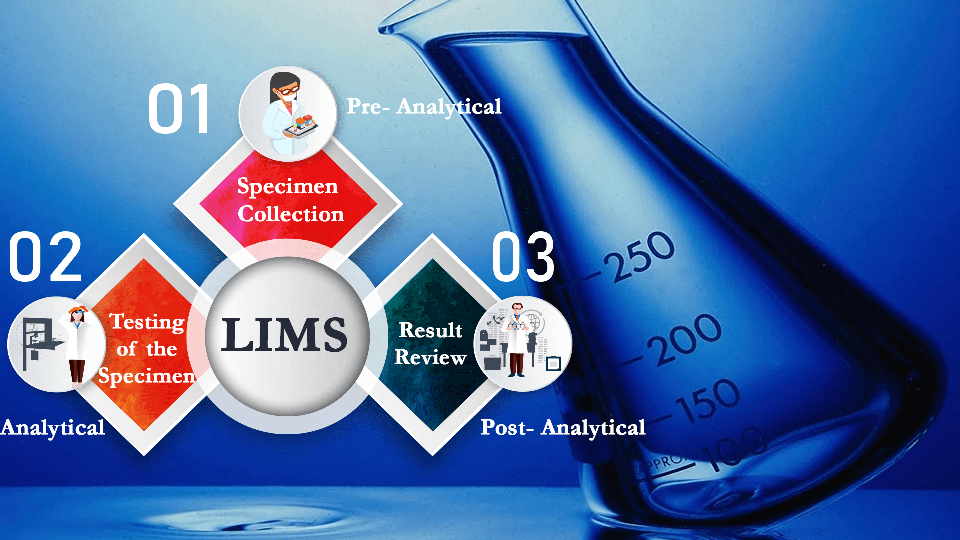
LIMS initiates meticulous sample tracking from the moment a sample is collected. It records clinical reports, phenotypic information, and even freezer locations in great detail. This comprehensive tracking covers freeze and thaw cycles, collection centre details, and responsible clinicians or lab technicians. With LIMS, samples are guaranteed to remain accurate and traceable throughout the entire process, ensuring the highest quality standards.
Chapter 3: Efficiency and Workflow Optimization with LIMS
3.1. Faster Turnaround Time (TAT)
Manual handling of laboratory processes often leads to delays in delivering test results to patients and clinicians. LIMS streamlines these processes, reducing errors and the need for cross-verification. It also efficiently manages and calibrates connected medical instruments, automatically updating them in response to alarming readings. This enhanced efficiency significantly improves the overall Turnaround Time (TAT).
3.2. Hassle-Free Workflow
LIMS takes the hassle out of managing laboratory workflow. The software automates workflow and records maintenance, saving valuable time. As processes are codified within the system, LIMS guides technicians on instrument usage and process handling. This reduces manual interventions for record movement and tracking, making the entire process hassle-free.
3.3. Streamlined Workflow for Doctors and Patients
LIMS offers dedicated login portals for doctors and patients. With preset templates for generating and sending invoices, it streamlines the workflow efficiency. Additionally, doctors and patients can easily access and compare current reports with previous ones, making diagnosis and treatment decisions more efficient.
Chapter 4: Productivity and Quality Control with LIMS
4.1. Enhanced Productivity
LIMS significantly boosts productivity in laboratory settings. It automatically shares lab test results across users, eliminating the need for manual distribution. Quick access to patient history is another productivity booster. Reports are synchronized with physicians' Electronic Medical Records (EMR) for rapid access. Automatic updates and alerts are generated for abnormalities, reducing the need for manual intervention. Furthermore, LIMS enables digital signatures, further cutting down on wait times for approving physical documents.
4.2. Quality Control Assurance
Maintaining quality in laboratory testing is paramount, and LIMS excels in this regard. Through effective Quality Control measures, identifying variations becomes easier. Quality Control results are stored as a database and can be compared to actual results, providing complete control over deviations or errors. With accreditations like NABL and others, LIMS ensures the highest quality standards in results and workflow.
The Future of Laboratory Information Management System(LIMS)
LIMS software is at the forefront of transforming laboratory management. Whether you operate a pathology lab, clinical research facility, or any other type of laboratory, investing in LIMS is a decision that promises to revolutionize your operations. Its integration of advanced technology, error reduction, streamlined workflow, enhanced quality control, and patient-focused features make it an indispensable tool in the healthcare industry. Embrace LIMS to stay ahead of the curve, enhance precision, boost efficiency, and deliver top-notch results while ensuring patient satisfaction.
With LIMS, the future of lab management is brighter than ever. It's time to invest in the future and take your laboratory operations to new heights with this powerful tool. Take advantage of the opportunity to be at the forefront of modern laboratory management. Invest in LIMS today, and reap the benefits of precision, efficiency, and quality.
Related Articles
Why Machine Interfacing in LIM...
As labs are updated and become more modern, better..... Read more
Is Cloud-Based SaaS Laboratory...
In every industry, modern laboratories are under p..... Read more
What is laboratory information...
..... Read more
Complex machine integration wi...
Save time on complex machines at your lab with Moc..... Read more