MocDoc's Offerings
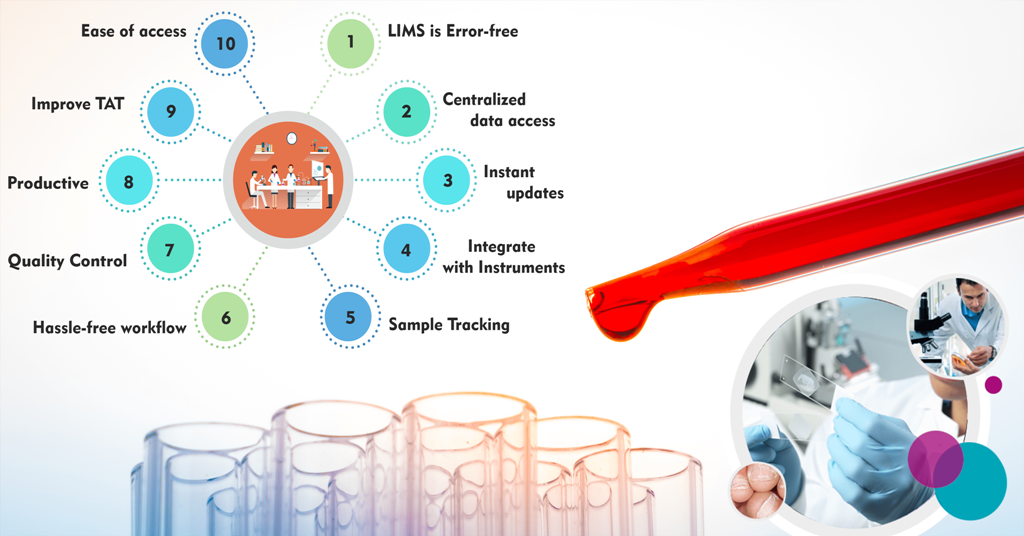
10 Signs You Should Invest in Lab Information management system
Published By
Sanjana
2019101512:46:38
Category LIMS

The rise of new technologies has made the volume of data drastically increasing day by day. The data volume generated globally across clinical research, biobanks, and other relevant testing laboratories are developing at a faster rate. Surviving the vast data volume and influencing it for research or business is stimulative for labs, especially if they don't possess a regulated mechanism to use and process the data.
One of the crucial reasons to invest in the Lab Information Management System (LIMS) is that it is used for organizing the data disregarding what type of industry you belong to. Information availability and rapid data growth have resulted in the extensive evolution of data management. Below are ten vital signs that state why you should invest in a LIMS.
1. Your laboratory holds higher operational costs:
Leading the particular portion of the allotted funds into laboratory personnel for management and data entry operations.
A Lab Information Management System frees human resources from time consuming and unproductive tasks, and therefore, they are considered as the high saver for your data entry and management operations. You will be able to pay-as-you-go on any basis like monthly or yearly if you prefer to go with a cloud-based LIMS. This means that you no need to make any investments towards hardware installations.
2. You hold transcriptional data entry errors and are not able to share information (data silos):
It indicates poor data traceability, problems with connecting data across various data sources & instruments, and data's manual input leading to transcriptional errors.
A Lab Information Management System combines with other data sources and instruments flawlessly and manages the data in the laboratory in an enhanced manner. LIMS automated calculations save time and decrease errors for laboratory personnel. Apart from this, the information can also be retrieved accurately at any time.
3. When there is a risk in your laboratory data:
The sign is when you are not able to grab access to perceptive clinical information, no reliability, not able to follow the regulations based on your industries, making use of paper-based systems and spreadsheets to manage data in your laboratory.
A Lab Information Management System provided enhanced reliability and data security by matching the industry-related standards, namely 21 CFR Part 11, HIPAA, and more. It also protects perceptive clinical information by hindering user access of unauthorized persons.
4. When your laboratory has an inferior disaster management system:
Insufficiency of a suitable mechanism for data storage and retrieval in the event of theft, fire, or natural calamities.
A Lab Information Management System ensures data continual by copying data to mirror servers, storing data on the primary servers, and also make sure on the timely backups. The information can be managed in case of any theft, fire, or natural disasters.
5. When you don't have real-time data metrics:
The sign indicates when there are no updates to laboratory clients and users on sample processing stages when there are no notifications and alerts for sample expiry or inventory depletion, and when there is no real-time display of metrics of the laboratory in the main dashboard to watch out important problems.
A Lab Information Management System alerts you regarding the inventory depletion or assists in identifying expired samples with the help of alarms, and this makes sure about the timely inventory replenishment. The LIMS also helps you to stay updated on testing or processing samples in your labs by sending application notification or email alerts so that you will be able to know the critical problems instantly.
6. When your laboratory has poor traceability and limited data storage capacity:
The sign is when there are any difficulties in tracing the possible data instantly by searching over different piles of paper data when generating an extensive amount of sample information with the help of paperwork, and when there are too many piles of paper data in the labs, limiting the capacity in the data storage.
A Lab Information Management System assists in managing information electronically and helps to decrease paperwork. You can accurately and instantly trace information with the help of different tools. A cloud-based LIMS protects the data in the cloud, and this data is accessible in real-time and 24/7 available anywhere.
7. When your laboratory is unable to reach the expected productivity:
Delayed Turnaround Times and experimental analysis over industry averages, and influencing human resources for auditing, manual data entry, report generation are the vital signs.
A Lab Information Management System helps labs to plan data management effectively, accelerate operations, and execute different business decisions. It also helps in generating rapid reports and control an audit trail along with time stamp and date.
8. When you have sustainability problems:
When your competitors receive more orders when compared to you and when there are insufficient business agility and responsiveness.
A Lab Information Management System can help in making your laboratory business active amid the complicated global market.
9. When your laboratory lacks collaboration scientifically:
When there requires a high sample turn around time and data duplication for sharing with collaborators and partners.
A Lab Information Management System, particularly in the case of cloud-based, helps in decreasing errors, avoid data duplication, enables accelerated turn around time, and effective collaboration. Apart from this, it also allows us to achieve higher profit, market share, and productivity.
10. When you have no capabilities in customizing for your different data management methods:
Applying new changes to the existing data manually to the older records, and where there is no customizations option available with spreadsheets and paper-based systems.
A Lab Information Management System enables customization as per your requirements and is also considered as the highly configurable solution. Any new changes can be applied instantly on the original records and therefore saves more time.
I hope the above signs let you choose the right LIMS Software. Any other queries and comments on the Lab Information Management System are welcome.
Related Articles
Why Machine Interfacing in LIM...
As labs are updated and become more modern, better..... Read more
Why should you invest in a Lab...
A Laboratory Information Management System (LIM..... Read more
7 Tips for handling a Producti...
Running a productive lab smoothly is tedious. Whet..... Read more
Is Cloud-Based SaaS Laboratory...
In every industry, modern laboratories are under p..... Read more