MocDoc's Offerings
Using LIMS for Quality Control Process
Published By
Sanjana
2018082215:58:39
Category LIMS

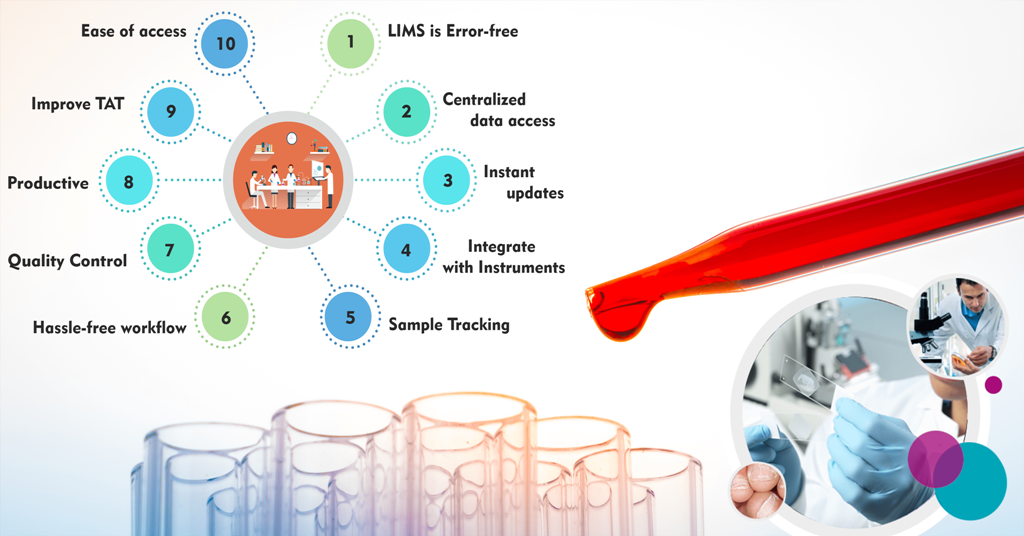
Keeping up with the fast-evolving world of trends means that smart work with innovation is required. Having a smart infrastructure built on informatics solutions can benefit the lab by implementing automation. Laboratory Information Management System (LIMS) is a critical part of the system to be incorporated in all functioning laboratories for better results. The LIMS of today has gone far beyond just sample management invoking functions like resource management, workflow management, and many more. By adhering to the National Accreditation Board for Testing & Calibration Laboratories (NABL) standards, labs can manage NABL quality indicators easily.
Quality control in Laboratory Information Management System (LIMS) Software:
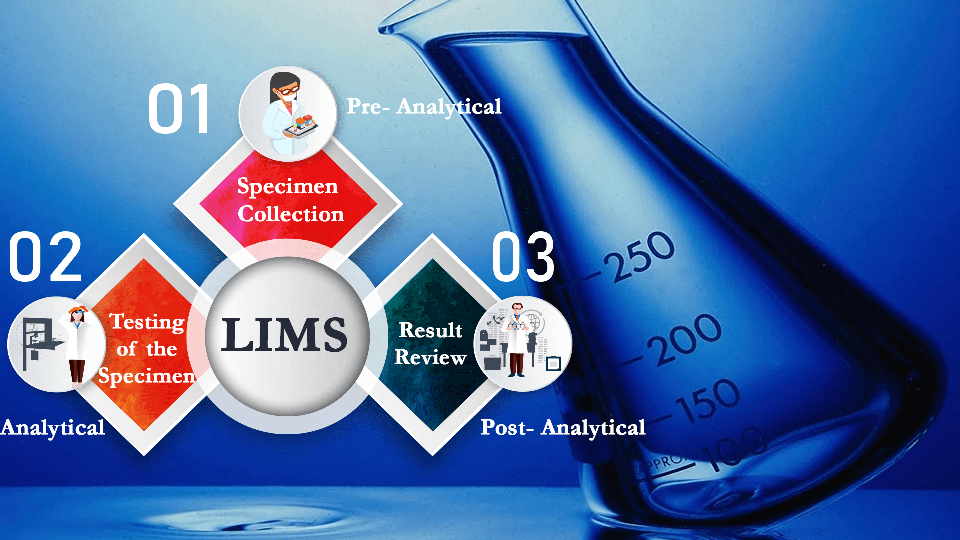
Quality control in LIMS Software is the process of assurance of quality at every stage included in the automation system. It may also be called a statistical process used to evaluate the analytics produced in the patient’s results. The sampling of the product is continued after every successful stage until the end of the sample management process. Quality control is essential in LIMS used in clinical laboratories as the result cannot be tampered with. Quality control is done on three major factors in a clinical lab, which are:
- Quality control on the sample
- Quality control of the utilized equipment
- Quality control for the process
For quality control of the sample, the result might either be quantitative or qualitative. This means that the result can either be a number count (hemoglobin count in the blood) or can define if the test result is positive or negative or can be limited to a few different values alone.
Quality control checks on the equipment or instrument are used to validate whether the instrument is operating within pre-defined specifications, inferring that patient test results are reliable. Once this validation is completed, the result of the patient can be used for further diagnosis.
Sample management:
Sample management is the primary process of a LIMS system. Any QC lab needs to analyze many kinds of samples depending on the industry of the laboratory. Our QC laboratory helps in the analyses of samples in a unique and distinct method. We have the following salient features for sample management:
- Single-sample management and multi-sample management by using templated management techniques.
- Sample recognition field that can easily identify the sample and log in the details, minimizing manual data entry.
- Barcoded labels for sample tracking.
- Implemented workflow automation features for reduced confusion and increased productivity.
Preparation and Method Execution:
- We have predefined execution methods for sample tracking, analysis of tracking response, complaint reception, reducing the dead time, and overall increasing the efficiency of our system.
- Special features for execution such as content uniformity, dissolution, stability, etc.
- Strict preparation and method execution policies like standardization, tracking of reagents, control of the environment, trainees, and equipment analysis.
Report Management:
We understand the importance of report generation and sensitivity in handling the report data. Hence we have assembled the following features for report management:
- Instant visualization of any abnormal notifications.
- Configurable testing methodologies and calculation techniques for managing high variant products.
- Multiple level approvals with comment entries for sensitive results.
Specialized Reporting:
- Flexible facilities that are fully configurable to the product and customer level including layout, content, units, significant digits, etc.
- Bi-directional integration with SAP-QM or SAP Batches for finished product testing. Interfaces to other ERP/MES systems are available.
- Streamlined delivery of event notifications and results to end-users via e-mail, fax, etc.
Decision Support:
The documents like SOP, analytical, maintenance, etc. are version-controlled for ISO standard compliance.
Features:
As important as quality control is in laboratories, so is analyzing the proper features best suited for every laboratory and making use of them appropriately. Some important Quality control features are as follows:
- Latest and updated versions of specifications are available on request.
- Multi-level product specification management is made with controlled releases.
- Sample management, sample storage, and reserve management are all done with accuracy.
- Sample planning with the batch-wise process and test plan management is done.
- Templated sample management by templating the sample at registration is effectively carried out for sample tracking.
- Quality control is done at multi-level, multi-departmental, and multi-premises.
- Workflow management with work schedule according to work plan for analysts and instruments alike.
- Accuracy in result capture and OOT management.
- Quality control on instruments by instrument calibration and regular maintenance scheduled at different time intervals with a proper update on status lock for instruments.
- Training on the new releases and sample management for every instrument is carried out for every lab personnel
- Any deviations from the original workflow are promptly recorded and validated.
- Stability in the study module is maintained.
LIMS System quality control process allows managing the data of products, samples, and instruments alike regularly by monitoring at various levels. This ensures that quality can be monitored and, if necessary, actions to improve quality.
Related Articles
Why should you invest in a Lab...
A Laboratory Information Management System (LIM..... Read more
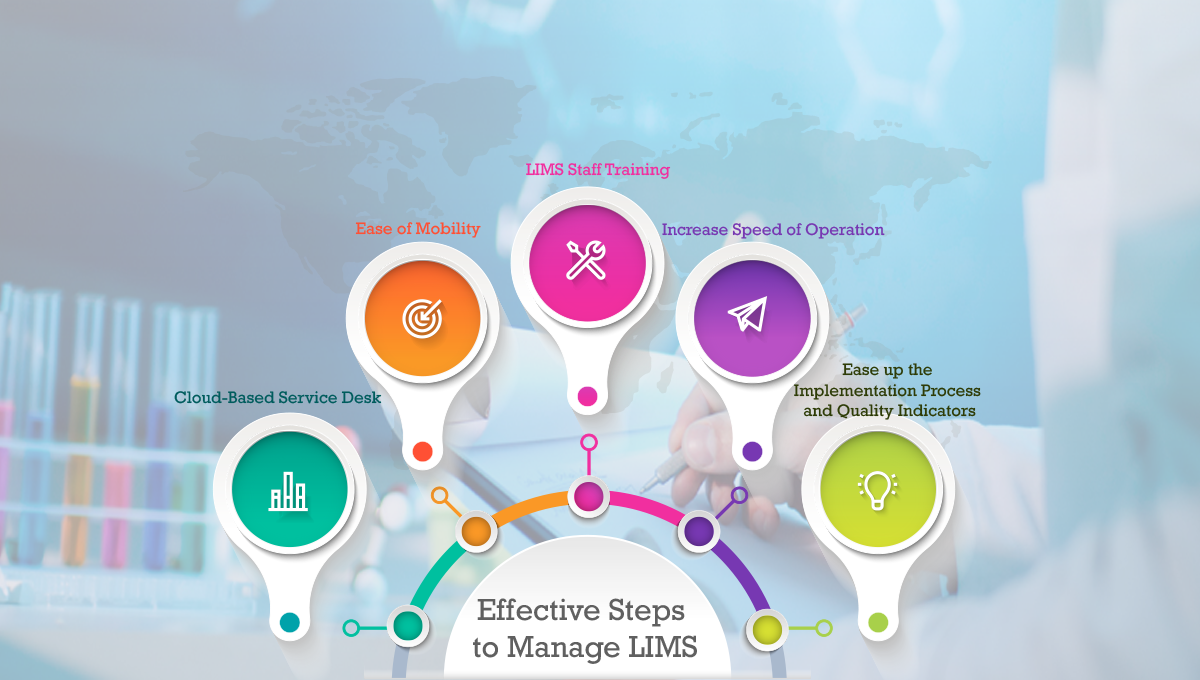
5 Effective Steps to Manage LI...
Effective Steps to Manage Laboratory Information M..... Read more
Streamlining Sample Tracking w...
Laboratory Information Management System is ..... Read more
What is laboratory information...
..... Read more