MocDoc's Offerings
5 Effective Steps to Manage LIMS
Published By
Sanjana
2018082415:59:00
Category LIMS
Effective Steps to Manage Laboratory Information Management System (LIMS) Software
Upgrading your clinical laboratory might be the latest enhancement you are implementing in your lab. This upgrade means you are automating the key functions to easily maintain the workflow. To make sure that your systems work in a smooth, harmonized, and safe manner, consider installing a cloud-based Laboratory Information Management system in your Lab. The cloud-based LIMS is no doubt the best choice to make as it has many advantages. A few of these are:
- The data stored in the cloud are fully encrypted, hence 100% data safety.
- Cost saving as there is no need for server maintenance.
- With effective data recovery, any data loss can be reverted.
- No manpower is required as everything is stored in the cloud, unlike physical servers.
Below are some simple tips to effectively manage your Cloud-based Laboratory Information Management System:
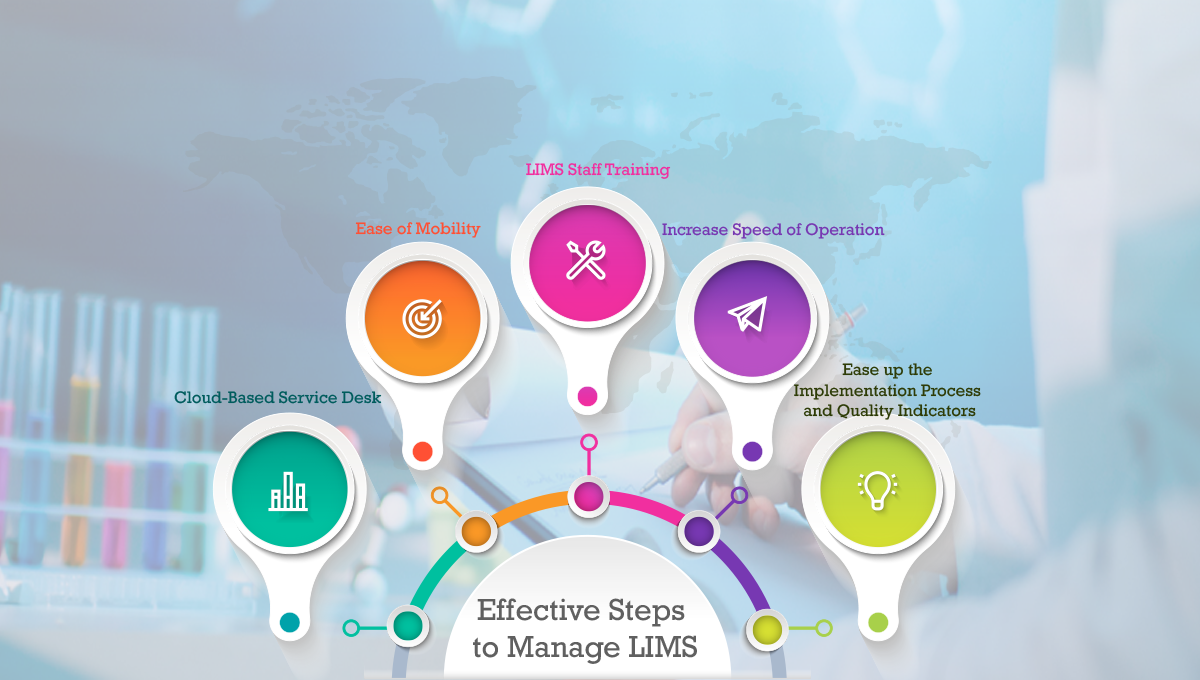
Implement a Cloud-Based Service Desk:
The most important step to do while implementing a cloud-based LIMS system is to provide a mini web page for clients to find information that caters to the needs of every patient. The internet is the influence king of the virtual world; so most of your patients will visit your website first, before heading over to your lab. Set up a cloud-based service desk and assist your patients just like you would do in your physical laboratory premises.
Make sure the webpage has a live chat facility so your customers can chat about the types of tests they can do and laboratory locations. Providing a live chat facility can help you to follow up in case the customers log out of the page suddenly. Also, features such as patient portals and customer portals empower your customers to download reports through the website making them completely independent.
Ease of Mobility:
Investing in a LIMS mobile app or website is the biggest advantage that Cloud-based servers can provide a laboratory. Since the data is stored at a remote location and is not bound by the constraints of physical hardware on the premises, investing in a mobile version of your laboratory’s website or even an App is a good idea.
You can take patient satisfaction to another level when you offer your patients and potential patients the ease to reach or access their health information on the go. With a virtual service desk in place, going mobile would mean more and better interactions and increased patient satisfaction.
LIMS Software Staff Training:
It’s essential to train laboratory staff to unlock the full power of your LIMS software application. To utilize LIMS software effectively, you can segregate the employees, departments wise, and provide training accordingly. Delegating one person as the head of training in every department can take the responsibility of providing LIMS-related knowledge sharing and periodic training. LIMS software training can include configuration options, storage, and retrieval of data, managing your workflows, and reporting. Staff training in each department is very important for businesses to succeed.
Increase Speed of Operation:
LIMS Software, specifically designed for laboratory information management, plays a pivotal role in optimizing operations. With a focus on pathology lab software, LIMS efficiently monitors various aspects such as Sample monitoring, sample workflow processes, patient visit count, inventory flow, cash flow, consultant performance, and overall business fluctuations over time. Through the analysis of these metrics, LIMS issues performance-based documents and develops standard quality indicators and key performance indicators, providing a comprehensive solution for laboratory management.
Additionally, Cloud-based LIS (Laboratory Information System) enhances the speed of laboratory operations. While technology facilitates remote meetings, Cloud-based LIS goes a step further by enabling access to data from any location, granting the user the necessary access rights. This feature ensures a swift exchange of information, contributing to an overall increase in the speed of laboratory operations. By seamlessly connecting personnel across different locations, the Cloud-based LIS becomes a valuable asset in streamlining workflows and promoting efficiency within the laboratory environment.
Ease up the Implementation Process and Quality Indicators:
The constant check of quality to ensure stability in business is a major requirement for any industry. With clinical laboratory, it is more of a service quality check rather than product one. Hence, quality indicators are of utmost importance to a laboratory.
LIMS Software, specifically designed for laboratory information management, can efficiently monitor various aspects of laboratory operations such as Sample monitoring, sample workflow process, patient visit count, inventory flow, cash flow, consultant performance, and overall business fluctuations over a period. The system analyzes these metrics to issue performance-based documents, and through the utilization of cloud technology, LIMS can develop standard quality indicators and key performance indicators.
By integrating a cloud-based LIMS System into your laboratory functions, you can optimize processes and identify areas for improvement. This innovative solution allows you to identify and understand your business metrics, paving the way for enhanced business optimization through the power of cloud technology. Whether it's managing sample workflows or evaluating consultant performance, the inclusion of LIMS Software, tailored for pathology lab software, brings a comprehensive and efficient approach to managing laboratory operations.
Related Articles
Why Machine Interfacing in LIM...
As labs are updated and become more modern, better..... Read more
Streamlining Sample Tracking w...
Laboratory Information Management System is ..... Read more
Revolutionize B2B sample colle...
MocDoc’s Runnr mobile/web application is designe..... Read more
10 Signs You Should Invest in ...
The rise of new technologies has made the volume o..... Read more