MocDoc's Offerings
What are the criteria to choose the best Laboratory Information System
Published By
Sanjana
2018081712:24:50
Category LIMS

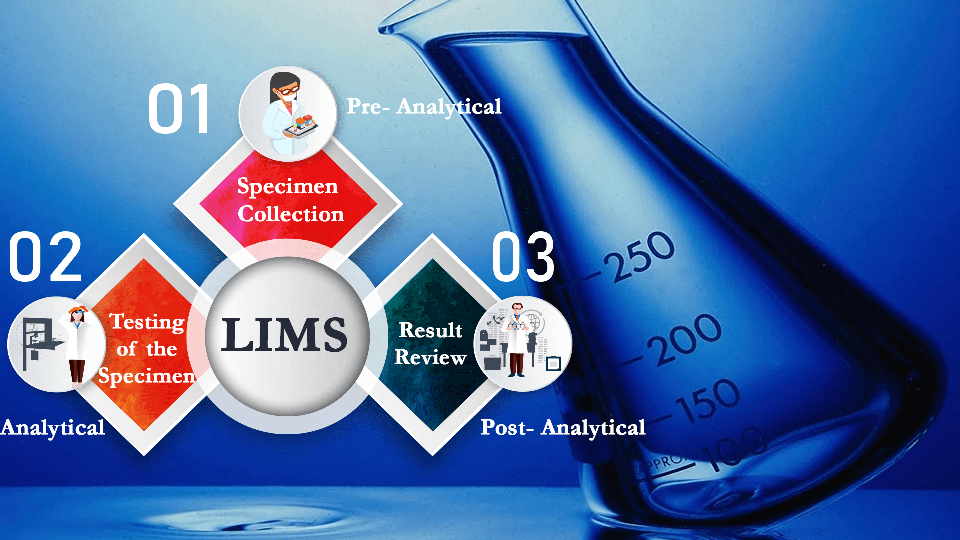
Laboratory Information Management System (LIMS) is a software-based solution that is used in labs for data management and to process a large number of lab samples to manage laboratory workflow. Evaluating and choosing the right LIMS must be done after analyzing some salient features like cost, interface, the ability of the system to evolve based on your future requirements, etc.
In this blog, we will help you evaluate the best and most cost-effective laboratory information system that will suit your requirements in the best way.
What to Choose Between Online and Offline Model of LIMS?
The offline model of LIMS software is an outdated one. Some disadvantages of using offline LIMS are:
- Not user-friendly.
- No mobile app facilities
- Reports can be generated. But patients have to get the reports directly by visiting the premises.
- The patient's record is not synchronized with multiple lab locations
- In case of a server crash, there is no possibility of getting data back. Less data security.
- Future technology updates are tough to install
- Maintenance is high.
Offline LIMS has some defects. Due to its unavailability online 24/7, the data collected can be stored only in a desktop, increasing the chances of data loss. The offline LIMS is best suited for small laboratories with only one clinical lab.
Why is the Online Model of LIMS the Best Choice?
The online model of LIMS is based totally on the cloud. Hence the data can be viewed from anywhere in the world if they are updated on the cloud. Monitoring live instrument data with the right tools will allow your company to make faster decisions with accurate information and support the on-time delivery of reports.
A time-consuming, manual process will be transformed into a fast, automated process, where human errors in collecting and processing information will be reduced to zero, allowing the company to concentrate its resources on its core functions and attain higher productivity and efficiency in its work.
Some benefits of online LIMS are:
- Low Maintenance cost.
- Instrument data storage for auditing purposes.
- An interface that works with any Database or LIMS.
- Get future updates as notifications
- Secure environment
- Track updates with the mobile app
Choosing The Right LIMS For You
Choosing the right Laboratory Information system depends purely on the requirement of the lab and the processes involved in it. You have to know the exact way in which the user data has to be logged in and the ways of analysis. This way you can easily find the exact LIMs software that would suit your requirements the best. Some of the requirements of a laboratory include:
- Functional requirements
- IT requirements
- Interface requirements
- Data management requirements
- Error handling
It is best advised to have business flow diagrams that will clarify the workflow, requirements, and processes in the lab. This business flow diagram will help in considering the instruments and systems involved in the lab that need to be interfaced with the software for harmonized workflow.
Understanding the Functionality Requirements
Understanding the requirements of your laboratory systems cannot be explained enough as the utmost important feature for choosing the right type of LIMS. A LIMS software used for the mining of oil and gas cannot be used completely for clinical purposes. Before investing in LIMS software from any vendor, it is vital to check that the software completely fulfills the needs of the laboratory. This includes the process of sample collection and data entry to the process of report generation and saving of reports. The functional requirements should be clearly listed and cross-checked regularly even after implementation in the system.
Some of the functional requirements that need to be checked in a LIMS are:
- Laboratory Test Processing.
- Test scheduling.
- Sample Tracking.
- Instrument control.
- Report generation.
- Quality control and Quality assurance.
- Statistical analysis.
- Resource management.
- Laboratory Safety and Accident Prevention Techniques.
Once the use cases that include the kind of user type, sample logging method, and result publishing are quantified, your functional requirements will get a lot clearer paving you for the best suitable LIMS purchase.
LIMS Future Upgrades
Choosing LIMS after a thorough analysis of your current needs is the best way of making an apt choice. However, the future of laboratory management might evolve with the changing technologies and more convenient workflow. Keeping that in mind, choose software from a vendor that is best suited for both your current needs and all your future needs.
Seeking advice on the improvement of the system might be too late when the implementation of LIMS software has already taken place. Jot down your requirements clearly and analyze the same with your team of experts who can justify the need for improvement based on futuristic updates.
When businesses want to add more locations, tie up with B2Bs, add a home collection module, NABL policy changes, the LIMS software should be able to upgrade and adopt it.
This will ensure that your business is on speed with the technology involved and can easily benefit from it in terms of productivity and workflow management.
LIMS Mobile Applications
Using LIS is the best method for the automation of Laboratory technologies. This implies that the process of computing sample analyses must be done faster, and more efficiently and there is easy workflow management with mobile apps.
Types of LIMS mobile apps:
- Home Collection App
- Runner App
- Laboratory Owner App
- B2B App
- Patient App
LIS helps pathologists approve data from anywhere in the most efficient system and can be extremely useful to labs and doctors alike. This saves a lot of time on the workflow. Automation of doctor approval in a mobile app can help LIS make more decisions quickly.
These were some basic points that might help you choose the best-suited LIS for your laboratory. Choose cost-effective software for your lab, but don’t miss on the quality or vendor credibility while choosing the right LIS for your laboratory.
24/7 Support System
When you choose LIMS software, make sure you are provided with 24/7 support facilities. When you face any issues with the LIMS software, you can contact the support professionals anytime and from anywhere and get the issues resolved quickly.
Data Security in LIMS
Security of data is the most basic need for any kind of industry; especially one involving the data and reports of patients. Implementation of a LIMS is specifically done to make the process easier and more secure, safeguarding the patient information from misuse. This has to be done while improving the knowledge level in the organization about the system by sharing the lab information accessible across various departments globally in the organization.
We have more information for you at Mocdoc - Digital Healthcare
Related Articles
Using LIMS for Quality Control...
Keeping up with the fast-evolving world of trends ..... Read more
What is laboratory information...
..... Read more
Why Machine Interfacing in LIM...
As labs are updated and become more modern, better..... Read more
Why only online-based LIMS wil...
Every modern industry laboratories are under inten..... Read more