MocDoc's Offerings
How MocDoc Helped Aruna Lab to Reduce the Workload
Published By
Santhosh
2019041114:21:47
Category LIMS

- Automated machine interfacing
- Integrated billing
- Improved rate plan
- Increase in patient count & revenue
- Reduced errors in billing
- Reduction in overall transportation cost
- High security with no 3rd party access
Related Articles
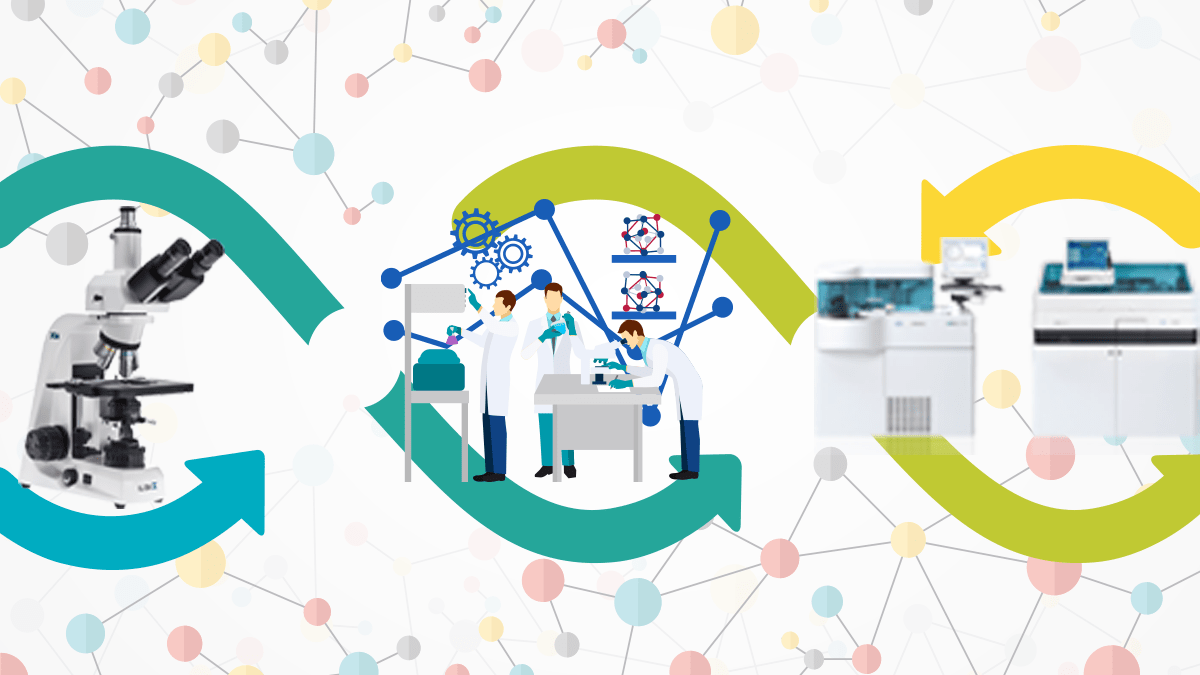
Types of Machine Interfacing in Laboratory Information Management System(LIMS):
Uni-Directional Instruments:
Bi-directional Instruments:
The Need to Interface Instruments with LIMS Software:
Increase in Productivity by Effective Machine Utilization:
Increase in Data Quality by Reducing Manual Errors:
Saves Time in output generation thereby satisfying the patients:
Why Machine Interfacing in LIM...
As labs are updated and become more modern, better..... Read more

A Laboratory Information Management System (LIMS): Why Do You Need One?
The adoption of Laboratory Information Management System (LIMS) has become increasingly crucial. This comprehensive guide delves deep into the reasons behind investing in LIMS, shedding light on the myriad benefits it offers to laboratory operations. As we explore the multifaceted facets of LIMS, you'll discover how this powerful tool revolutionizes lab management, enhancing precision, efficiency, and overall quality. Whether you're running a pathology lab, clinical research facility, or any laboratory, this article is your key to understanding why LIMS is a game-changer.
Chapter 1: LIMS Software - A Paradigm Shift in Lab Management
In this digital age, traditional manual processes in laboratories have become a bottleneck, prone to errors and inefficiencies. LIMS software, also known as a laboratory management system, emerges as a game-changer, transforming how laboratories operate. It encompasses a wide range of functionalities, including ERP tools, data analytics capabilities, and virtual software. Let's explore why LIMS is gaining ground in modern laboratories.
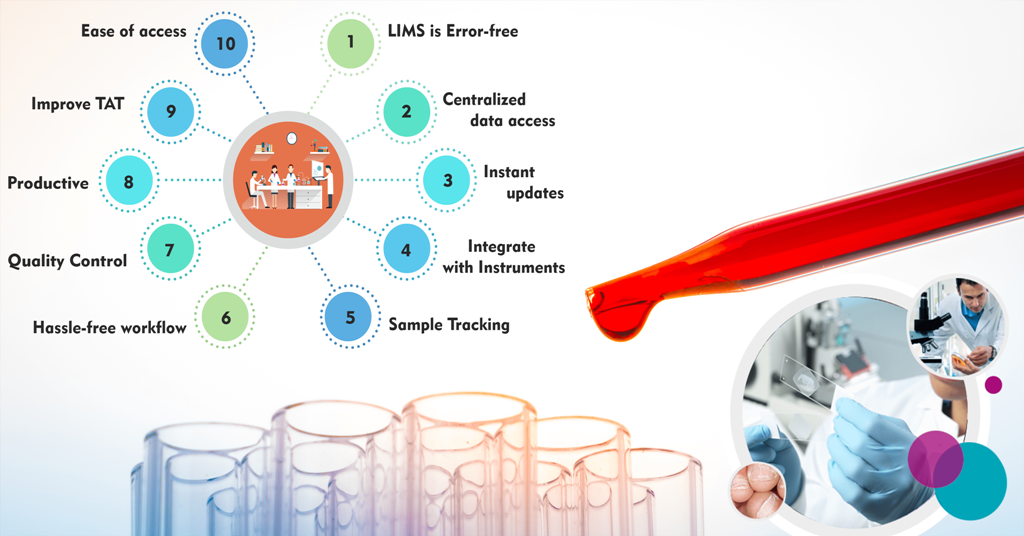
1.1. Error-Free Precision
LIMS software is designed to be virtually error-free, a crucial feature for laboratories dealing with sensitive data. Manual handling of laboratory tasks often leads to data mismatches, incorrect readings, and erroneous updates. LIMS ensures impeccable record precision, eliminating these sources of error.
1.2. Centralized Data Access
One of the standout features of LIMS is its ability to provide centralized data access. This means that patient records and reports are easily accessible to authorized personnel. Clinicians, technicians, and other stakeholders can quickly reference and verify patient details. Moreover, LIMS automatically updates critical values and responds to crises, ensuring immediate attention to critical cases.
1.3. Seamless Integration with Instruments
LIMS seamlessly integrates with clinical instruments, streamlining the testing process. It automatically retrieves and stores test results in its primary database, eliminating the need for manual data entry. This not only reduces the risk of errors but also saves valuable time.
Chapter 2: Enhancing Patient Experience through LIMS
2.1. Patient-Focused Updates
LIMS introduces a new level of patient engagement by providing real-time updates. Patients no longer need to anxiously wait for their test results. Instead, they receive automatic updates via SMS or email, along with concise test result summaries. This not only enhances patient satisfaction but also ensures they are well-informed about the progress of their tests.
2.2. Sample Tracking for Quality Assurance
LIMS initiates meticulous sample tracking from the moment a sample is collected. It records clinical reports, phenotypic information, and even freezer locations in great detail. This comprehensive tracking covers freeze and thaw cycles, collection centre details, and responsible clinicians or lab technicians. With LIMS, samples are guaranteed to remain accurate and traceable throughout the entire process, ensuring the highest quality standards.
Chapter 3: Efficiency and Workflow Optimization with LIMS
3.1. Faster Turnaround Time (TAT)
Manual handling of laboratory processes often leads to delays in delivering test results to patients and clinicians. LIMS streamlines these processes, reducing errors and the need for cross-verification. It also efficiently manages and calibrates connected medical instruments, automatically updating them in response to alarming readings. This enhanced efficiency significantly improves the overall Turnaround Time (TAT).
3.2. Hassle-Free Workflow
LIMS takes the hassle out of managing laboratory workflow. The software automates workflow and records maintenance, saving valuable time. As processes are codified within the system, LIMS guides technicians on instrument usage and process handling. This reduces manual interventions for record movement and tracking, making the entire process hassle-free.
3.3. Streamlined Workflow for Doctors and Patients
LIMS offers dedicated login portals for doctors and patients. With preset templates for generating and sending invoices, it streamlines the workflow efficiency. Additionally, doctors and patients can easily access and compare current reports with previous ones, making diagnosis and treatment decisions more efficient.
Chapter 4: Productivity and Quality Control with LIMS
4.1. Enhanced Productivity
LIMS significantly boosts productivity in laboratory settings. It automatically shares lab test results across users, eliminating the need for manual distribution. Quick access to patient history is another productivity booster. Reports are synchronized with physicians' Electronic Medical Records (EMR) for rapid access. Automatic updates and alerts are generated for abnormalities, reducing the need for manual intervention. Furthermore, LIMS enables digital signatures, further cutting down on wait times for approving physical documents.
4.2. Quality Control Assurance
Maintaining quality in laboratory testing is paramount, and LIMS excels in this regard. Through effective Quality Control measures, identifying variations becomes easier. Quality Control results are stored as a database and can be compared to actual results, providing complete control over deviations or errors. With accreditations like NABL and others, LIMS ensures the highest quality standards in results and workflow.
The Future of Laboratory Information Management System(LIMS)
LIMS software is at the forefront of transforming laboratory management. Whether you operate a pathology lab, clinical research facility, or any other type of laboratory, investing in LIMS is a decision that promises to revolutionize your operations. Its integration of advanced technology, error reduction, streamlined workflow, enhanced quality control, and patient-focused features make it an indispensable tool in the healthcare industry. Embrace LIMS to stay ahead of the curve, enhance precision, boost efficiency, and deliver top-notch results while ensuring patient satisfaction.
Why should you invest in a Lab...
A Laboratory Information Management System (LIM..... Read more

Quality control in Laboratory Information Management System (LIMS) Software:
- Quality control on the sample
- Quality control of the utilized equipment
- Quality control for the process
Sample management:
- Single-sample management and multi-sample management by using templated management techniques.
- Sample recognition field that can easily identify the sample and log in the details, minimizing manual data entry.
- Barcoded labels for sample tracking.
- Implemented workflow automation features for reduced confusion and increased productivity.
Preparation and Method Execution:
- We have predefined execution methods for sample tracking, analysis of tracking response, complaint reception, reducing the dead time, and overall increasing the efficiency of our system.
- Special features for execution such as content uniformity, dissolution, stability, etc.
- Strict preparation and method execution policies like standardization, tracking of reagents, control of the environment, trainees, and equipment analysis.
Report Management:
- Instant visualization of any abnormal notifications.
- Configurable testing methodologies and calculation techniques for managing high variant products.
- Multiple level approvals with comment entries for sensitive results.
Specialized Reporting:
- Flexible facilities that are fully configurable to the product and customer level including layout, content, units, significant digits, etc.
- Bi-directional integration with SAP-QM or SAP Batches for finished product testing. Interfaces to other ERP/MES systems are available.
- Streamlined delivery of event notifications and results to end-users via e-mail, fax, etc.
Decision Support:
Features:
- Latest and updated versions of specifications are available on request.
- Multi-level product specification management is made with controlled releases.
- Sample management, sample storage, and reserve management are all done with accuracy.
- Sample planning with the batch-wise process and test plan management is done.
- Templated sample management by templating the sample at registration is effectively carried out for sample tracking.
- Quality control is done at multi-level, multi-departmental, and multi-premises.
- Workflow management with work schedule according to work plan for analysts and instruments alike.
- Accuracy in result capture and OOT management.
- Quality control on instruments by instrument calibration and regular maintenance scheduled at different time intervals with a proper update on status lock for instruments.
- Training on the new releases and sample management for every instrument is carried out for every lab personnel
- Any deviations from the original workflow are promptly recorded and validated.
- Stability in the study module is maintained.
Using LIMS for Quality Control...
Keeping up with the fast-evolving world of trends ..... Read more

In every industry, modern laboratories are under pressure to enhance operational efficiency, decrease costs, control regulatory compliance, and improve quality. Apart from this, the rise of CRO (Contract Research Organization) and R&D externalization strategies in the industry of pharmaceuticals has led to laboratories gathering enormous data from different partners, preceding a significant increase in the complexity of lab workflow.
LIMS (Laboratory Information Management System) and informatics Solutions are being used in the pharmaceutical industry to meet these challenges. The main reason why cloud-based LIMS is preferred is due to its advanced features like
● Decreased cost,
● Automatic software updates,
● Disaster and backup recovery services,
● Rapid implementation of LIMS,
● Flexibility,
● Security,
● Accessibility,
● Collaborations.
One of the successful Laboratory solutions and laboratory report software used by most users is SaaS. Let's explore the SaaS in depth below.
What is SaaS?
SaaS(Software as a Service), is one of the software distribution models and types of cloud computing like IaaS and PaaS. The third-party provider hosts the program rather than downloading and installing the software to run on your PC. Once the software is hosted, it's accessed over the internet by users via a web user interface.
Why SaaS, a cloud-based LIMS, is perfect for your lab?
SaaS, pathology management software provides plenty of excellent benefits that make it the preferred option of every lab. According to the Forrester Research Inc. survey held last year, 15% of SMB (Small to Medium Businesses) and 16% of the larger organizations make use of SaaS. Compared to the previous year's research, the larger enterprise has seen a 33% increase in the Usage of SaaS. Considering the smaller enterprise, they have seen a 50% increase in SaaS. Below are some reasons to state why Cloud-based LIMS is the right option for your laboratory.
Hosting Options:
Few providers provide the private cloud option that can host within your facility. The private cloud option offers different SaaS LIMS benefits but with fewer security risks. A cloud is public; either it can be private, single-tenant, or multi-tenant. It's easier to validate the single-tenant cloud when compared to the multi-tenant as they are less prone to intrusion or data leakage.
Data Integrity and Audit Trails:
A permanent audit trail is vital for addressing different concerns in data integrity, both from the perspective of product and regulatory quality. You need to ensure that your SaaS LIMS holds a detailed and reliable audit trail for every piece of information. You also need to make sure that the data is maintained continuously on the various servers rather than on the data itself.
Apart from that, you need to ensure that your provider has set their system default to store a completely reliable and flexible audit trail. It also controls the network logs and detailed server. It offers data center third-party auditing insurance. You need to ensure your SaaS LIMS is capable of integrating with the other informatics systems and with your instruments.
Service Level Agreement:
You must develop a Service Level Agreement with your provider who will be able to address all of the above-concerned areas and more. A Service Level Agreement that is well written is capable of making the variation between a smooth execution & roll out of your SaaS time overruns, cost, and a few of the unexpected expenses incurred.
Security:
When dealing with a SaaS Laboratory Management System, you will depend upon your providers to secure your pieces of information as it is going to be stored in the cloud. Almost every cloud service provider offers excellent security for your valuable data.
Even though the data breach risk is less, few organizations that belong to heavily regulated areas hesitate to enable their information to move away from their firewall. Make sure you have evaluated the policies of your security thoroughly. It means you have your data secured adequately before working with SaaS LIMS.
Validation:
The important compliance responsibility with every regulation resets with the service provider or manufacturer. Most of the vendors advertise these cloud-based SaaS LIMS as a prevalidated system. But this refers to the network of cloud providers. You need to ensure that you get clear about the meaning of the prevalidated system by the vendor. Also, you need to know what scope is covered so that you can know your system is executed in the right way in the user environment.
Apart from that, SaaS LIMS cloud providers handle every clinical laboratory software patch and upgrade. It ensures that you utilize the system's recent version. You need to understand who should manage revalidation once there is an upgrade or patches take place.
Business Continuity:
You need to understand how data recovery and backup can be handled, and also explore who is the reason and responsible for it. The details of the guarantee like service, response time, hardware, uptime, etc. provided by the provider should be stated clearly in the SLA (Service Level Agreement). Apart from that, the Service Level Agreement should contain the details of recovery and backup services that are included. Also, it should hold the cost incurred by exploring a few of the optional services.
System Failover:
There can't be anyone who didn't use Google. For instance, what will happen in case a rogue asteroid crashes into the server farms of Google? Nothing happens. Airtight failover technology and Mirrored servers indicate that the news service will display the crater's aerial photos, which once acted as the server farm. High availability and uptime are included in the terms & conditions of the cloud deals that are negotiated in any form.
Instrument Interfacing:
The complexity of the SaaS LIMS is challenging. Private, hosted, and On-Premises cloud LIMS is capable of handling instrument interfacing in the same manner. It is difficult to understand the SaaS LIMS cloud providers' ability, track record, and philosophy when you deal with the instrument interface.
Economics of Scale:
SaaS LIMS cloud providers provide you with more or less due to the technology's specialized use. As many customers utilize identical resources at the same cost, data centers, are slashed, and then the savings are passed to the customer.
Bottom Line:
In the past few years, technology providers have developed cloud-based SaaS LIMS based on market demand. SaaS LIMS enables to facilitate enhanced scalability, agility, innovation, and connectivity across the lab environment. It helps to increase efficiency in operation as well as decrease cost. The decision to make use of a cloud-based SaaS LIMS will be taken into step by a mix of various concerns like regulatory, operations, and security. The best way to ensure that your corporation benefits the higher from a SaaS approach to a laboratory information management system is to work with a top-notch informatics consultant.
Is Cloud-Based SaaS Laboratory...
In every industry, modern laboratories are under p..... Read more